IMARC Group has recently released a new research study titled “South Korea biosimilar market Size, Share, Trends and Forecast by Component, Deployment Mode, SMS Traffic, Application, End User, and Region, 2025-2033”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
South Korea Biosimilar Market Overview
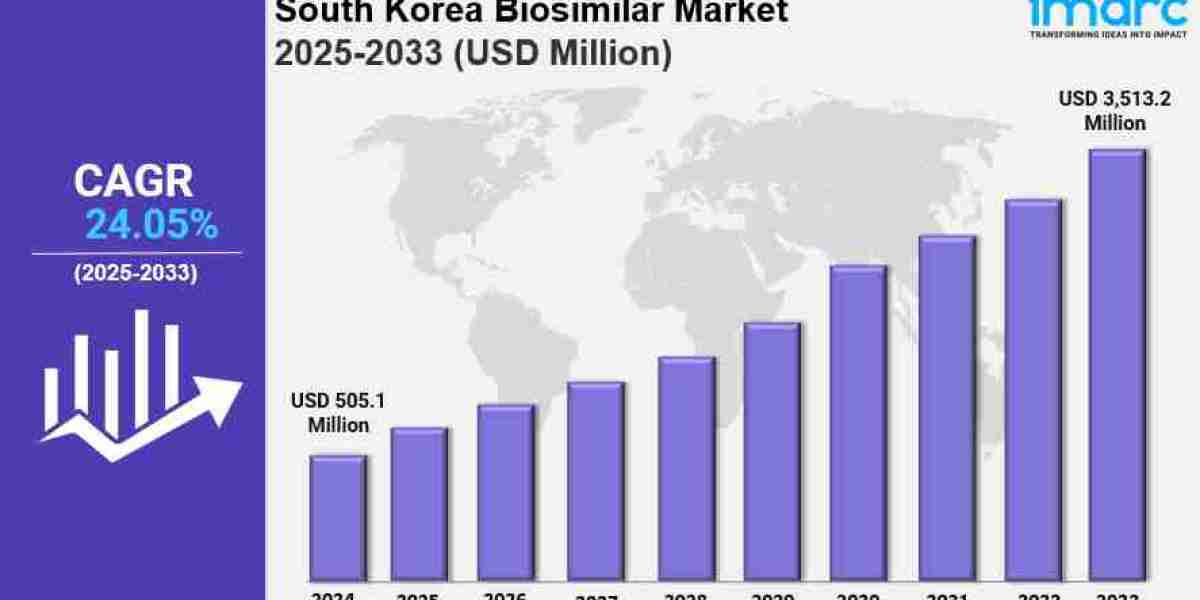
The South Korea biosimilar market growth was valued at USD 505.1 Million in 2024. Looking forward, IMARC Group estimates the market to reach USD 3,513.2 Million by 2033, exhibiting a (CAGR) of 24.05% from 2025-2033.
Market Size and Growth
Forecast Years: 2025-2033
Historical Years: 2019-2024
Market Size in 2024: USD 505.1 Million
Market Forecast in 2033: USD 3,513.2 Million
Market Growth Rate (2025-2033): 24.05%
Request for a sample copy of the report: https://www.imarcgroup.com/south-korea-biosimilar-market/requestsample
Key Market Highlights:
✔️ Robust market growth driven by rising demand for cost-effective biologic therapies
✔️ Expanding healthcare access and aging population fueling biosimilar adoption
✔️ Strong regulatory support and streamlined approval processes boosting market entry
✔️ Increasing R&D investment and partnerships enhancing local biosimilar production
Trends in the South Korea Biosimilar Market
The South Korea biosimilar market forecast is set to experience several transformative trends that will shape its future landscape. One notable trend is the growing emphasis on patient education and awareness regarding biosimilars. By 2025, the South Korea biosimilar market size is expected to grow as healthcare providers and manufacturers invest in educational initiatives to inform patients about the safety, efficacy, and cost benefits of biosimilars. This increased awareness is crucial for overcoming misconceptions and building trust in biosimilar therapies.
Additionally, the rise of partnerships and collaborations between pharmaceutical companies and research institutions is becoming more prevalent, leading to accelerated development and commercialization of new biosimilars. The South Korea biosimilar market share is likely to reflect this collaborative approach, as joint efforts enhance innovation and streamline the development process. Furthermore, advancements in technology, such as biomanufacturing and analytical techniques, are improving the quality and consistency of biosimilars, further driving market growth.
These trends highlight the evolving dynamics of the biosimilar market in South Korea, positioning it for sustained growth and innovation in the coming years.
Market Dynamics of South Korea Biosimilar Market
Increasing Demand for Cost-Effective Biologics
The South Korea biosimilar market is experiencing substantial growth driven by the increasing demand for cost-effective biologics. As healthcare costs continue to rise, both patients and healthcare providers are seeking affordable alternatives to expensive biologic therapies. Biosimilars, which are highly similar versions of approved biologic medicines, provide a viable solution by offering comparable efficacy and safety at a lower price point. By 2025, the South Korea biosimilar market size is expected to expand significantly as more biosimilars receive regulatory approval and enter the market. This shift is further supported by government policies aimed at promoting the use of biosimilars to enhance access to essential medications and reduce healthcare expenditures. As awareness of the benefits of biosimilars grows, their adoption in therapeutic areas such as oncology, rheumatology, and endocrinology is likely to increase, driving market growth.
Regulatory Support and Evolving Guidelines
Another key dynamic influencing the South Korea biosimilar market is the robust regulatory support and evolving guidelines established by health authorities. The South Korean government has implemented a comprehensive regulatory framework that facilitates the development and approval of biosimilars, ensuring that they meet stringent safety and efficacy standards. By 2025, the South Korea biosimilar market share is anticipated to grow as regulatory bodies streamline the approval process, making it easier for companies to bring biosimilars to market. This supportive environment encourages investment in biosimilar research and development, fostering innovation and competition within the industry. Furthermore, ongoing collaboration between regulatory agencies and industry stakeholders helps to establish clear guidelines for biosimilar development, addressing concerns related to interchangeability and pharmacovigilance. As a result, the South Korea biosimilar market is well-positioned for growth, driven by a favorable regulatory landscape.
Expanding Therapeutic Applications
The South Korea biosimilar market is also benefiting from the expanding therapeutic applications of biosimilars across various medical fields. Initially focused on oncology and autoimmune diseases, the scope of biosimilar applications is broadening to include other therapeutic areas such as ophthalmology, cardiology, and infectious diseases. By 2025, the South Korea biosimilar market size is expected to reflect this diversification as new biosimilars targeting different indications receive regulatory approval. This expansion not only enhances treatment options for patients but also allows healthcare providers to manage a wider range of conditions more effectively and affordably. Additionally, the increasing number of biosimilars entering the market is fostering competition, which contributes to lower prices and improved access to biologic therapies. As the demand for innovative treatments continues to rise, the biosimilar market in South Korea is poised for significant growth, driven by the expanding range of therapeutic applications.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=39266&flag=C
South Korea Biosimilar Market Segmentation:
The market report segments the market based on product type, distribution channel, and region:
Molecule Insights:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Indication Insights:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Regional Insights:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
Competitive Landscape:
The market research report offers an in-depth analysis of the competitive landscape, covering market structure, key player positioning, top winning strategies, a competitive dashboard, and a company evaluation quadrant. Additionally, detailed profiles of all major companies are included.
Key Highlights of the Report
1. Market Performance (2019-2024)
2. Market Outlook (2025-2033)
3. COVID-19 Impact on the Market
4. Porter’s Five Forces Analysis
5. Strategic Recommendations
6. Historical, Current and Future Market Trends
7. Market Drivers and Success Factors
8. SWOT Analysis
9. Structure of the Market
10. Value Chain Analysis
11. Comprehensive Mapping of the Competitive Landscape
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers create lasting impact. The firm offers comprehensive services for market entry and market expansion.
IMARC’s services include thorough market assessments, feasibility studies, company formation assistance, factory setup support, regulatory approvals and license navigation, branding, marketing and sales strategies, competitive landscape and benchmark analysis, pricing and cost studies, and sourcing studies.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302