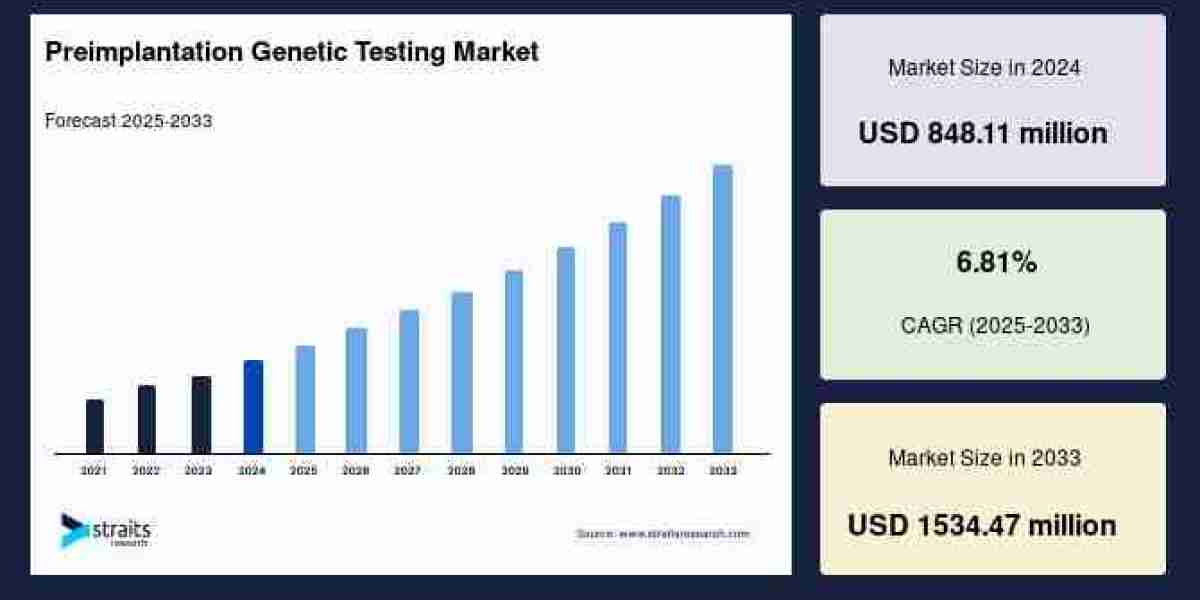
The cell therapy manufacturing market is experiencing rapid expansion due to the increasing adoption of regenerative medicine, technological advancements in cell processing and scaling, and the growing prevalence of chronic and genetic diseases worldwide. In 2024, the global market was valued at USD 4.24 billion and is projected to reach USD 16.71 billion by 2034, growing at a CAGR of 14.70% during 2025–2034.
Cell therapy manufacturing involves producing therapeutic cells that are used to repair or replace damaged tissues and cells in the body. These therapies have shown exceptional potential in treating conditions such as cancer, autoimmune disorders, cardiovascular diseases, and neurodegenerative diseases. The market is expanding as both pharmaceutical companies and academic institutions increase investment in cell-based research and commercial production.
Overview of the Cell Therapy Manufacturing Market
Cell therapy manufacturing refers to the complex, highly regulated process of cultivating, expanding, and modifying cells for therapeutic use. It includes several stages—cell isolation, genetic modification, expansion, harvesting, and quality control testing. The goal is to ensure that the final therapeutic product meets strict safety and efficacy standards before administration to patients.
The market is being driven by the rising success of CAR-T cell therapies, stem cell therapies, and gene-modified cell treatments. Regulatory approvals for advanced therapies and the increasing establishment of Good Manufacturing Practice (GMP) facilities are also boosting market growth.
Moreover, the ongoing evolution in automation, bioprocessing technologies, and artificial intelligence (AI)-driven cell analytics has significantly enhanced production scalability and cost efficiency.
Request sample report: https://www.expertmarketresearch.com/reports/cell-therapy-manufacturing-market/requestsample
Cell Therapy Manufacturing Market Size and Share
The cell therapy manufacturing market size was valued at USD 4.24 billion in 2024, with North America holding the largest share due to the presence of major biotech firms, strong regulatory frameworks, and a robust clinical pipeline for advanced therapies.
By 2034, the market is projected to reach USD 16.71 billion, reflecting widespread adoption across oncology, cardiovascular, and orthopedic applications.
Key insights:
T-cell therapies dominate the therapy type segment, driven by the growing success of CAR-T therapies for hematologic malignancies.
Viral vector technology accounts for a major share in the technology segment due to its crucial role in genetic modification.
The autologous cell source currently leads, but allogeneic therapies are expected to gain momentum due to lower production costs and scalability advantages.
Cell Therapy Manufacturing Market Dynamics and Trends
Market Drivers
Rising prevalence of chronic diseases:
Increasing cases of cancer, autoimmune disorders, and cardiovascular diseases are fueling demand for personalized and regenerative treatment options.Technological innovations in cell processing:
Advances in automation, 3D bioreactors, and closed-system manufacturing are improving efficiency and product quality.Regulatory support and funding:
Governments and regulatory agencies, such as the FDA and EMA, are providing fast-track designations for innovative therapies, accelerating product approvals.Growth in clinical trials:
A significant rise in the number of cell therapy clinical trials globally underscores growing confidence in these treatments’ therapeutic potential.
Market Restraints
High manufacturing and processing costs.
Complex regulatory procedures for therapy approval.
Limited standardization in cell culture and testing methods.
Emerging Trends
Shift toward allogeneic "off-the-shelf" therapies to reduce costs and improve scalability.
Adoption of AI and machine learning for optimizing manufacturing workflows.
Growth in contract development and manufacturing organizations (CDMOs) specializing in cell and gene therapy production.
Cell Therapy Manufacturing Market Growth Outlook (2025–2034)
The cell therapy manufacturing market growth trajectory remains robust, supported by increased public and private investments, rapid technological advancement, and rising success rates in clinical trials.
North America will continue to dominate the market, driven by its innovation-driven ecosystem and favorable regulatory support. However, Asia Pacific is expected to witness the fastest growth due to rising healthcare spending, expansion of biomanufacturing facilities, and increasing adoption of advanced cell therapy technologies in countries like Japan, China, and South Korea.
Europe remains another significant hub due to its well-established biotech infrastructure and supportive regulatory pathways for regenerative medicine development.
Market Opportunities and Challenges
Opportunities
Expansion of manufacturing capabilities: Growing demand for commercial-scale facilities presents a lucrative opportunity for contract manufacturers.
Partnerships and collaborations: Increased strategic alliances between biotech firms and CDMOs to enhance manufacturing capacity.
Adoption of automation and AI: The integration of digital technologies will improve process consistency, reduce contamination risk, and optimize cell yield.
Challenges
Regulatory hurdles: Navigating diverse global regulatory frameworks remains complex.
Supply chain limitations: Dependence on high-quality raw materials and specialized reagents can disrupt production.
High R&D costs: The long development cycle and high initial investment may limit entry for small and medium enterprises.
Recent Developments in the Cell Therapy Manufacturing Market
November 2024: Novartis AG expanded its CAR-T cell therapy production facility in Switzerland to meet growing demand.
September 2024: Thermo Fisher Scientific launched an automated, closed-system platform for large-scale cell therapy production.
July 2024: Lonza Group partnered with Bluebird Bio Inc. to enhance vector manufacturing for gene-modified cell therapies.
May 2024: Catalent, Inc. announced the expansion of its cell therapy manufacturing capacity in the United States.
March 2024: Merck KGaA introduced a next-generation viral vector technology platform designed to increase transfection efficiency.
These developments underline the industry’s focus on capacity expansion, process innovation, and strategic collaboration to accelerate therapy commercialization.
Competitive Landscape and Key Players
The cell therapy manufacturing market is moderately consolidated, with major players focusing on innovation, acquisitions, and partnerships to strengthen their global presence.
Key Companies Covered:
Novartis AG
F. Hoffmann-La Roche AG
Gilead Sciences, Inc.
Thermo Fisher Scientific, Inc.
Catalent, Inc.
JSR Life Sciences LLC (KBI Biopharma Inc.)
Waisman Center (Waisman Biomanufacturing)
Cell and Gene Therapy Catapult
Merck KGaA
Lonza Group
Oxford Biomedica Plc
WuXi AppTec
Charles River Laboratories International Inc.
Institut Mérieux (ABL Inc.)
BioCentriq
Centre for Commercialization of Regenerative Medicine (CCRM)
Fujifilm Holdings Corporation (Fujifilm Cellular Dynamics)
Amgen Inc.
Bluebird Bio Inc.
Takeda Pharmaceutical Company Limited
These companies are investing heavily in R&D, biomanufacturing infrastructure, and global partnerships to expand their capacity and support commercialization of next-generation therapies.
Cell Therapy Manufacturing Market Segmentation
By Therapy Type
T-Cell Therapies
Dendritic Cell Therapies
Tumour Cell Therapies
Stem Cell Therapies
Others
By Technology
Somatic Cell Technology
Cell Immortalization Technology
Viral Vector Technology
Genome Editing Technology
Cell Plasticity Technology
3D Technology
Others
By Source of Cell
Autologous Cell
Allogeneic Cell
By Scale of Operation
Preclinical
Clinical
Commercial
By Application
Oncology
Cardiovascular Diseases
Orthopaedic Diseases
Others
By End User
Pharmaceutical and Biotechnology Companies
Academic and Research Institutes
Others
By Region
North America
Europe
Asia Pacific
Latin America
Middle East and Africa
Frequently Asked Questions (FAQs)
1. What is the Cell Therapy Manufacturing Market, and how big is it?
The cell therapy manufacturing market involves the production of therapeutic cells for regenerative and immunotherapy applications. It was valued at USD 4.24 billion in 2024 and is expected to reach USD 16.71 billion by 2034, growing at a CAGR of 14.70%.
2. What factors are driving the growth of the Cell Therapy Manufacturing Market?
Key growth drivers include the increasing prevalence of chronic diseases, advancements in bioprocessing and automation, and growing investments in regenerative medicine.
3. Which technology segment dominates the Cell Therapy Manufacturing Market?
Viral vector technology holds the largest market share due to its critical role in gene-modified cell therapies such as CAR-T treatments.
4. What challenges does the Cell Therapy Manufacturing Market face?
The market faces challenges such as high production costs, complex regulatory processes, and supply chain constraints related to cell culture materials.
5. Who are the major players in the Cell Therapy Manufacturing Market?
Prominent companies include Novartis AG, Thermo Fisher Scientific, Lonza Group, Merck KGaA, Catalent Inc., and WuXi AppTec, among others.