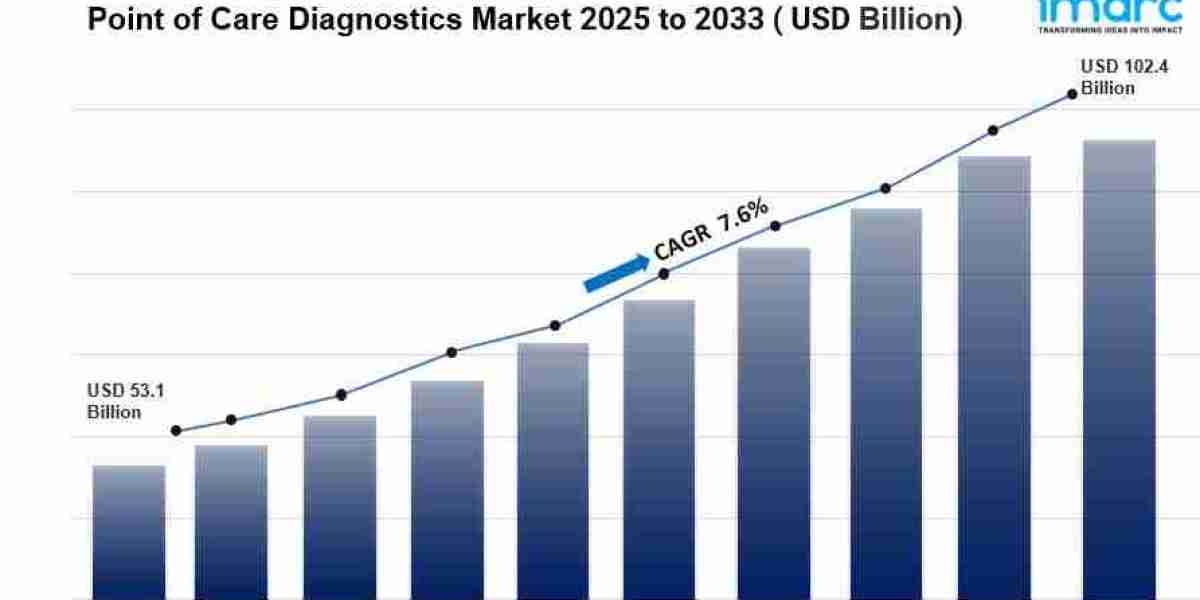
The global Point-of-Care Diagnostics Market was valued at USD 53.11 Billion in 2024. It is projected to grow to USD 102.4 Billion by 2033, reflecting a CAGR of 7.6% during the forecast period from 2025 to 2033. Key drivers include the increasing need for rapid, convenient diagnostics, rising prevalence of infectious diseases worldwide, and technological advancements in miniaturization and biosensors.
The global Point of Care Diagnostics Market Share is increasing steadily as healthcare systems prioritize rapid, on-site testing for faster clinical decision-making. Rising prevalence of chronic and infectious diseases, growing demand for home-based testing, and advancements in portable diagnostic technologies are key drivers of market expansion. Innovations in biosensors, microfluidics, and smartphone-integrated devices further support the growth of the Point of Care Diagnostics Market Size. Additionally, the shift toward decentralized healthcare, early disease detection, and improved patient outcomes continues to strengthen market demand worldwide.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
Point-of-Care Diagnostics Market Key Takeaways
- Current Market Size: USD 53.11 Billion in 2024
- CAGR: 7.6% during 2025-2033
- Forecast Period: 2025-2033
- The market is propelled by the prevalence of chronic and infectious diseases needing effective management through rapid and accurate testing.
- Increasing demand for rapid testing kits in emergency and remote settings is expanding market adoption.
- Integration of biosensors and microfluidics enhances diagnostic efficiency and reliability.
- Rising investments in healthcare infrastructure and positive regulatory initiatives promote product accessibility.
- The U.S. market is driven by growing chronic disease prevalence and home-based testing device demand.
Download a sample PDF of this report: https://www.imarcgroup.com/point-of-care-diagnostics-market/requestsample
Market Growth Factors
The global point-of-care diagnostics market is majorly driven by the rise in chronic and infectious diseases requiring faster and accurate diagnostic solutions. According to the World Health Organization, non-communicable diseases cause 41 million deaths annually, representing 74% of all deaths, while infectious diseases like HIV affect millions globally. The escalating incidence of diseases such as diabetes, cardiovascular disorders, and respiratory conditions necessitates convenient monitoring and early detection, particularly in remote and underserved regions where traditional laboratory access is limited. Point-of-care (POC) diagnostics meet these needs by offering rapid, effective testing to support disease management.
Technological advancements significantly bolster market growth by enabling development of smart, portable POC devices with enhanced accuracy and expanded test menus. For example, the development of portable RT-PCR devices identifying multiple viruses within 30 minutes and ultra-fast molecular tests delivering COVID-19 results in under 15 minutes exemplify this progress. Integration of biosensors, microfluidics, and AI-driven diagnostics further streamlines testing processes, improving reliability and user-friendliness. Collaborations among companies to innovate and commercialize compact devices with multiple testing capabilities are transforming healthcare delivery and accelerating market expansion.
The growing emphasis on personalized medicine represents another key growth driver. With initiatives like the National Cancer Institute’s precision medicine efforts and rising government healthcare expenditures reaching USD 9 trillion globally, patient-centric approaches demand rapid, targeted diagnostics. POC diagnostics play a critical role by enabling tailored treatment plans through precise disease identification and monitoring. As precision medicine gains widespread adoption, the confidence and acceptance of POC diagnostic solutions among patients and healthcare providers increase, further stimulating market demand.
Market Segmentation
Analysis by Product Type:
- Blood-Glucose Monitoring Kit: User-friendly kits that empower patients to self-test without specialized training, supporting widespread adoption, especially in remote areas. Expected to grow with diabetes cases projected to rise from 529 million to 1.3 billion by 2050.
- Cardio-Metabolic Monitoring Kit
- Pregnancy and Fertility Testing Kit
- Infectious Disease Testing Kit
- Cholesterol Test Strip
- Hematology Testing Kit
- Others
Analysis by Platform:
- Lateral Flow Assays: Simple, stable tests providing results via visual colored lines, requiring minimal training and small sample volumes. Supported by investments to develop advanced tests.
- Dipsticks
- Microfluidics
- Molecular Diagnostics
- Immunoassays
Analysis by Prescription Mode:
- Prescription-Based Testing: Tests ordered by healthcare professionals after regulatory approvals, essential for disease detection and management such as cancer and autoimmune conditions.
- OTC Testing
Analysis by End-User:
- Professional Diagnostic Centers: Facilities equipped with advanced equipment and trained staff performing diverse tests, preferred for comprehensive healthcare services.
- Home Care
- Research Laboratories
- Others
Regional Insights
North America leads the global point-of-care diagnostics market in 2024 due to its advanced healthcare infrastructure and prevalence of chronic diseases like diabetes and cardiovascular disorders. The region’s well-established medical facilities facilitate integration of POC diagnostics into routine care. For instance, CDC projects 1 in 3 to 1 in 5 Americans will have diabetes by 2050, driving demand for POC testing solutions.
Recent Developments & News
- April 2024: Roche Diagnostics India launched a PoC NT-proBNP test to screen diabetes patients at risk of cardiovascular diseases.
- December 2023: Thermo Fisher Scientific Inc. partnered with Project HOPE to expand access to HIV testing among HIV Positive Youth in Sub-Saharan Africa.
- January 2023: Beckman Coulter, Inc. formed a strategic partnership with MeMed to develop a host immune response diagnostic test.
Key Players
- Abbott Laboratories
- Beckman Coulter, Inc.
- Becton, Dickinson and Company
- F. Hoffmann-La Roche AG
- Instrumentation Laboratory
- Johnson & Johnson
- Nova Biomedical Corporation
- Pts Diagnostics
- Qiagen
- Siemens
- Trinity Biotech
Customization Note
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Ask An Analyst: https://www.imarcgroup.com/request?type=report&id=2113&flag=C
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302